How Heat Transfer By Conduction?
In this article, we will learn about the heat transfer mechanism in conduction, how heat transfer takes place by conduction, understand about thermal conductivity, units of thermal conductivity, look at thermal conductivity for metal, non-metals, and gases. Then, at last, understand thermal resistance and thermal resistance units.
- Heat transfer is a transfer of thermal energy due to temperature differences between bodies. If there is a temperature difference between the bodies, heat transfer will take place. In nature, heat is always transferred from high temperature to low temperature. The reverse can be achieved by applying some additional input work (Compressor in the refrigerator). The transfer of heat stops when the two bodies or mediums reach the same temperature. This condition is called thermodynamic equilibrium.
- Heat transfer plays a very important role in our life. It has countless applications. It is a phenomenon which keeps our home cools during summer days and hot during the winter days. Heat transfer prevents our car from overheating. It keeps our food fresh.
There are 3 modes of Heat transfer
- Conduction
- Convection
- Free convection
- Forced convection
3. Radiation
How Heat Transfer By Conduction?
- As a very fundamentally, Conduction is the transfer of energy (heat) from higher energetic particle to lower energetic particle via interaction between these particles at molecular levels. Conduction occurs in solid, liquid, and gases.
- Now, let’s understand the mechanism of heat transfer by conduction in solid, liquid, and gases. As we know, in solid, the molecules are very close to each other, and heat transfer takes place with the atomic activity in the form of lattice vibration due to electron interaction with lattice or due to electron interaction with each other. This interaction is completely random. Hence, heat can transfer in any direction from the heat generation point. Electronic interaction also helps in heat transfer. This is the reason, why a good electrical conductor is also a good thermal conductor.
- In gas, let’s Consider the gas is filled by two Surfaces. These surfaces are maintained at different temperatures. Due to that, a temperature difference occurs across the gas. Consider gas is stationary, not have any bulk motion, then the energy at any point in the gas can be transferred by internal rotational, random translation & vibration motion of the gas molecules. As expected, higher energetic molecules have high temperature this randomly molecular interaction lead to molecular collisions which transfer energy from higher molecules to lower energetic one.
- In the case of liquid, The heat transfer by conduction is the same as in a gas. In liquid, molecules are close to each other compared to gas, and molecules interactions are stronger than gases.
Fourier’s Law (Heat Transfer By Conduction)
- To describe the conduction behavior, Fourier’s law is used. It is described the relationship between heat flux (q) and temperature gradient. Mathematically, It is written as q = -K dt/dx
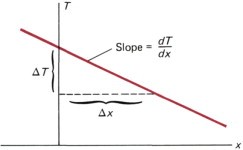
Why is there a negative sign in Fourier’s Law of heat conduction?
Where q = Heat flux
K = Thermal conductivity
- Consider an electric heater is ON, the amount of heat transfer by heater per unit time is called heat flow rate (Q). If we divide the heat flow rate with a cross-section area of the heater coil, we get heat flux (q).
- K is thermal conductivity. It is a material property that describes the ability of the material to transfer heat. The larger the thermal conductivity(K), the smaller the temperature gradient, and the faster the heat transfer occurs.
Now, assuming a bar with a uniform cross-section throughout the length L. The bar has two different temperatures at both ends. Assuming the temperature changes are linearly along the bar. Then, the heat flux can be written as
q = -K (T2 – T1)/L ————— (1)
As we know, q is heat flux, which means heat flow rate per unit area ( q = Q/A ). Replace the ‘q’ in the above equation (1). After mathematical rearrangement, we will get the equation…
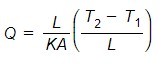 The ratio L/KA is known as thermal resistance. It is similar to electrical resistance. Electrical resistance describes the ability of a material to flow electricity. Thermal resistance indicates resistance faced by thermal energy during heat flow.
The ratio L/KA is known as thermal resistance. It is similar to electrical resistance. Electrical resistance describes the ability of a material to flow electricity. Thermal resistance indicates resistance faced by thermal energy during heat flow.
Let’s understand the significance of material properties thermal conductivity (K) and thermal resistance (L/KA) in detail.
Thermal conductivity (K)
- From our experience, some materials conduct the heat at a faster rate compared to other materials. For example, the electric heater is made from copper because copper conducts heat very fast compared to other metals. So that the heating coil can heat fastly in less time.
- The cooking panhandle is made from thermoplastic resin which conducts the heat at a very slow (almost negligible) rate. That’s why we can hold the pan during the cooking.
- The ability of different materials to conduct the heat is described by thermal conductivity. Thermal conductivity is a material property just like young modulus.
- In US customary unit system, the unit of thermal conductivity is BTU / hr-ft-f. Where BTU stands for British thermal unit. In the international system of units, the unit of thermal conductivity is W/mk.
- Generally, metals have very high thermal conductivity. Metals are very good conductors of heat. Nonmetals and gases have a low thermal conductivity which is acts as a good insulator of heat. The solid has the highest thermal conductivity. The gases have the lowest thermal conductivity and the liquid have a thermal conductivity in between solid and gases’ thermal conductivity. (Ksolid > Kliq > Kgas).
In solid, there are two types of solid.
- Crystallized solid – The solid which have a well-organized crystal structure.
- Amorphous solid – the solid which has a randomly-non organized crystal structure.
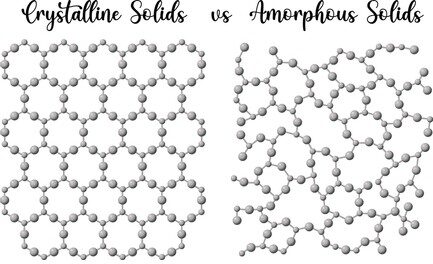
- Normally metals belong to crystallize solids that have a well-organized crystal structure. Nonmetals belong to amorphous solids.
- In an amorphous solid, the molecules have random motion between them due to that there are more voids between them, and voids are filled with air that’s why non-metals have lower thermal conductivity compared to metals. Except for the diamond and quartz which also belong to non-metals but they have a very good organization crystal structure and have good thermal connectivity.
- The second reason is, In metal, the number of free electrons is high while in non-metals number of free electrons is low. Due to the lack of free electrons, non-metals have poor thermal conductivity.
- In the case of alloy, Alloys have a lower thermal conductor compared to their parent material.
- In the metal range, silver has the highest thermal conductivity than copper and then gold (Ksilver > Kcopper > Kgold).
- Silver has the highest thermal conductivity material 416 W/mK. The most widely used metal, the thermal conductivity of aluminium is 237 W/mK.
Here is the list of thermal conductivity for metals, non-metals, and gases.
| Thermal conductivity of aluminum | 237 |
| Thermal conductivity of water | 0.6 |
| Thermal conductivity of copper | 399 |
| Thermal conductivity of gold | 317 |
| Thermal conductivity of iron | 80.2 |
| Thermal conductivity of carbon steel (1 %) | 43 |
| Thermal conductivity of stainless steel (1 %) | 15.1 |
| Thermal conductivity of glass | 0.81 |
| Thermal conductivity of plastic | 0.2-0.3 |
| Thermal conductivity of wood | 0.087 |
| Thermal conductivity of cork | 0.039 |
| Thermal conductivity of ethylene glycol | 0.26 |
| Thermal conductivity of hydrogen | 0.18 |
| Thermal conductivity of benzene | 0.159 |
| Thermal conductivity of air | 0.026 |
Now, let’s talk about variable thermal conductivity. It is possible that at different temperatures, the value of thermal conductivity is different means ‘K’ is not constant with temperature. Thermal conductivity (K) at a particular temperature can be calculated by
Kvariable = K0 (1 ± βT)
- K0 = thermal conductivity at 0° C
- β = constant term
- T = temperature
- Kvariable = K0 (1+βT) is used to find thermal conductivity in nonmetals. Similarly, Kvariable = K0 (1- βT) is used to find ‘K’ in metals.
In the case of non-metals, with an increase in temperature, the ‘K’ is also increasing. The reason is that non-metals are made from a material that has no organized crystal structure. Due to that, there are more voids between the molecules, and these voids are filled with gas. Now with the increment in temperature the kinetic energy of gas molecules is also increasing, due to the high kinetic energy of gas molecules, the number of collisions is increasing. As the collision increase, the gas will become more conducive this phenomenon is happening globally in voids of material at the same time which leads to an increase in the thermal conductivity of non-metal with an increase in temperature.
Thermal resistance
According to Fourier’s law,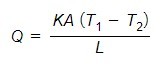
Let’s compare the above equations with an electrical analogy.
Where, I is replaced by ‘Q’, V is replaced by ‘T’ and Relc is replaced by Rth.
- According to ohm’s law,
Relc= V/I
I = V/Relc ———-(2)
- Compare equations 1 and 2, we noticed that Relc can be equal to L/KA which is known as thermal resistance Rth.
- Electrical resistance is a resistance felt by the electrical energy during the flowing from the circuit. Similarly, thermal resistance is a resistance felt by thermal energy during flowing from one surface to another surface. It indicates the magnitude of thermal resistivity.
- From equation…
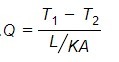
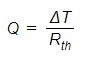
- From the above equation, as more temperature difference, the heat rate will be more. If the thermal resistance increases then the rate of heat transfer (Q) will decrease.
From the equation, Rth = L/KA
- The SI unit of thermal resistance is celcius/watt or Kelvin/watt.
- Thermal resistance is directly proportional to length (Rth α L). As more length (distance between two surfaces) the value of thermal resistance will also be large.
- Thermal resistance is inversely proportional to thermal conductivity (Rth α 1/K). If some material has good thermal conductivity then its thermal resistance will be low as thermal resistance is inversely proportional to thermal conductivity.
Based on that, we can make two categories.
- If thermal conductivity is high and thermal resistance is less, such type is known as ‘conductors’.
- If thermal conductivity is low and thermal resistance will be more, such type is known as ‘insulators’. Which takes more time to transfer the heat from one surface to another surface. As they have low thermal conductivity.



